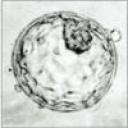
 Flip switch for stem cells — Three research teams reported a technique for “reprogramming” skin cells into embryonic stem cells, those primordial bits of protoplasm that can propagate themselves indefinitely and, under the right conditions, transform themselves into any type of cell in the body. Deriving embryonic stem cells normally requires destroying an embryo — the main reason research with the cells remains limited, as does federal support for the work.
Flip switch for stem cells — Three research teams reported a technique for “reprogramming” skin cells into embryonic stem cells, those primordial bits of protoplasm that can propagate themselves indefinitely and, under the right conditions, transform themselves into any type of cell in the body. Deriving embryonic stem cells normally requires destroying an embryo — the main reason research with the cells remains limited, as does federal support for the work.
Teams from Kyoto University, MIT and a Harvard-UCLA collaboration all confirmed a report last year out of Kyoto that skin cells could be reprogrammed by implanting four genes that produce proteins called transcription factors, which control the effects of other genes. Adding those four factors kicked off an intracellular chain reaction that reverted the skin cells to a primordial, “pluripotent” state characteristic of embryonic cells.
There are, of course, a number of caveats, the most significant of which is that the work so far has only succeeded in mouse cells. Extending it to human cells may be tricky, in part because they will likely require additional transcription factors. What’s more, at least one of the transcription factors used in the mouse experiments appears to contribute to cancer; so may the type of virus used to transfer the transcription-factor genes into skin cells. As a result, it’s not yet clear whether the embryonic stem cells produced this way could be used to help regenerate damaged tissue or organs in humans, such as dopamine-producing neurons for Parkinson’s patients or insulin-secreting pancreatic cells for diabetics. All that said, it’s a very encouraging step forward, both in terms of advancing understanding of stem-cell biology and the potential for altering the ethical landscape for the work.
For more, see the WSJ and the NYT.
The trials of Avandia, continued — The fracas over the controversial diabetes drug continued to throw shrapnel in all directions, leaving almost no one unscathed. Avandia’s maker, GlaxoSmithKline, published interim data from a large trial of the drug in the New England Journal of Medicine and touted it as evidence of the drug’s safety — only to find it accompanied by three editorials nitpicking the data and arguing that the data did little to allay doctors’ concerns. In congressional hearings today, a diabetes expert said he was berated by a company official and threatened with a lawsuit for raising questions about Avandia’s safety several years ago. Then the FDA commissioner told Congress that the agency would require strict new warning labels on Avandia and a similar drug.
The FDA, meanwhile, faced charges that one of its drug-safety officials was reprimanded after earlier recommending exactly those warnings for Avandia. And congressional Republicans went after Cleveland Clinic cardiologist Steven Nissen, who first raised the alarm about Avandia almost three weeks ago, suggesting that he had colluded with Democrats to embarrass the FDA and to enhance his chances of one day becoming FDA commissioner himself. As if all that weren’t enough, the NYT also asked if GSK’s efforts to promote Avandia to African-Americans may be backfiring.
Whew.
 In research, where you stand depends on who’s paying the bills — An unusual report in the journal PLoS Medicine found that research studies sponsored by drugmakers were 20 times more likely to favor the companies’ products than trials with no disclosed funding source. Conclusions drawn by the research scientists involved — apart from the data itself, that is — were 35 times more likely to favor a sponsor’s drug, the PLoS Medicine study found.
In research, where you stand depends on who’s paying the bills — An unusual report in the journal PLoS Medicine found that research studies sponsored by drugmakers were 20 times more likely to favor the companies’ products than trials with no disclosed funding source. Conclusions drawn by the research scientists involved — apart from the data itself, that is — were 35 times more likely to favor a sponsor’s drug, the PLoS Medicine study found.
The study looked at nearly 200 published trials of the cholesterol-lowering drugs known as statins to gauge what effect the source of funding had on the results. The research team noted that several factors could account for the tremendous slant in favor of sponsors’ products, including the fact that drug companies might squelch negative studies instead of publishing them and the possibility that trials might be deliberately designed in order to skew the results. For more, check out this San Francisco Chronicle story.
Cancer drug news — The year’s biggest cancer meeting, the annual gathering of the American Society for Clinical Oncology, took place in Chicago over the weekend. Here are a few highlights you might have missed:
- Meeting focus shifts from new drugs to new applications for old drugs (Business Week)
- New drugs show promise for liver cancer (WSJ)
- Biotech Telik reveals that its ovarian-cancer drug Telcyta not only didn’t work, it apparently killed women five months sooner than those receiving standard treatment; FDA orders halt to current Telcyta studies (Bloomberg, TheStreet.com)
California pension fund, private-equity money chase healthcare “cost-cutting” — Calpers, the largest pension fund in the U.S., invested $700 million in Health Evolution Partners, a private-equity fund run by a former Bush administration health official that aims to find ways to reduce the cost of healthcare. According to the NYT, the fund will focus on new ideas such as remote monitoring of elderly or demented patients in order to keep them out of nursing homes longer, telemedicine, chronic-disease management and genomics-based “personalized medicine” that tailors treatments to individual genetic profiles. One thing the fund will not be looking at, though, is electronic health records, which HEP founder David Brailer championed for two years in the Bush administration without much impact; he terms the field “a saturated market.” (See also this story in the SF Chronicle.)
I laid out some early thoughts on high-tech approaches to the healthcare crisis in this piece on Andy Grove’s reform crusade. In general, many of the things Brailer is interested in strike me much the same way Grove’s ideas did — that is, as worthy but woefully insufficient efforts if you expect them to actually reduce healthcare costs. Truly tackling the problem of costs means addressing structural failures of the system, including the current fee-for-service system that rewards doctors financially for doing more procedures or prescribing more drugs regardless of whether or not they help patients. In addition, it wouldn’t hurt to find out more about which medical treatments help patients most — which the NYT’s David Leonhardt addressed here and which I wrote about here — which probably also ultimately means restricting the use of the treatments deemed less effective.
Viewed against that backdrop, Health Evolution Partners and Calpers may be doing little more than spitting into the wind.
Speaking of healthcare reform — The WSJ weighed in with this piece on why the subject of healthcare reform doesn’t send politicians fleeing anymore. The usual suspects — rising health-insurance premiums and out-of-pocket costs, the growing numbers of uninsured Americans — make their obligatory appearance, but the really interesting nugget is how the insurance industry is angling to co-opt reform rather than opposing it outright as it did with the Clinton plan in 1993-94. That alone speaks volumes as to how thoroughly fed up many Americans have become with the ramshackle mess we call a healthcare system.
And while we’re on the subject — One of the major Bush administration healthcare-reform initiatives — besides the smashing success of electronic medical records, that is — was to push Medicare to institute financial incentives that would reward doctors for providing better care. Such “pay for performance,” however, seems to be falling short, according to a pilot study published in the Journal of the American Medical Association and described by the WSJ. In that study, 54 hospitals in the “pay for performance” plan did no better at improving medical practices than 446 hospitals that weren’t offered the incentives.
Gates to measure global public-health performance — No, not all by himself. The Bill and Melinda Gates Foundation, however, gave the University of Washington a $105 million grant to track the impact of public-health programs on a worldwide basis. From the WSJ article:
The new institute will be headed by Christopher Murray, a professor at the University of Washington, who previously served as director of a public-health program at Harvard University and as an official at the World Health Organization. The institute’s missions will include collecting and analyzing data on health trends, such as the prevalence of major diseases and the availability of health services, as well as conducting independent evaluations of the effectiveness of health programs.
The plan reflects the Gates foundation’s strategy of using statistical measures to determine the effectiveness of its grant-giving. The institute will disseminate the information it gathers about global health issues, programs and giving, officials said.
Institute officials pointed to the dearth of available statistics in global health, contrasting that to business, which runs on clear results. “It would seem strange that we would need an institute like this,” Dr. Murray said. But in public health, “we’re so far behind the norm in other sectors.”
The grant is the foundation’s second aimed at gathering better global public-health information.

